Challenge
A top-20 pharma needed to scale high-volume CSR authoring with a platform trusted for regulated content: one that delivers consistent quality and compounds improvement through human feedback and refinement.
Source Documents & Inputs
Style Guide
CSR Template
Study Protocol
Statistical Analysis Plan (SAP)
Tables, Figures & Listings (TFLs)
Storyboards
Data Interpretation Meeting (DIM) files
Solution
The company partnered with Peer AI on a staged rollout to validate quality, measure impact, and scale CSR authoring.
Proof of Concept (POC): Trial for Phase 1 and complex Phase 3 studies
Evaluation: Medical writer & editor review POC using predefined metrics
Production: Deployed Peer AI in production for Phase 1 CSRs
Implementation at scale: Peer AI deployment for all CSRs
Results & Impact
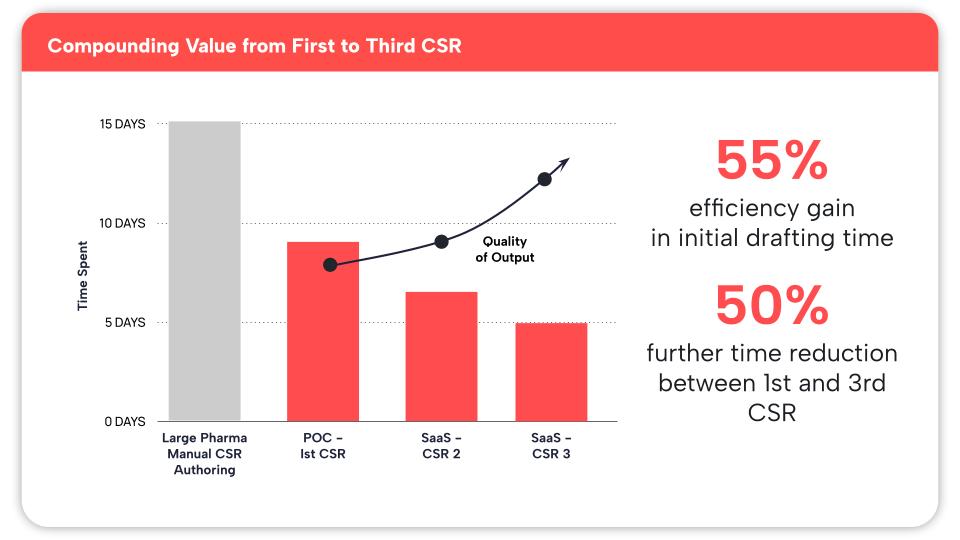
Initial Efficiency Gains: Achieved a 55% reduction in CSR drafting time compared with traditional authoring approaches
Compounding Improvement: Realized an additional ~50% reduction in time between the first and third CSR
Quality by Measured Criteria: Improved accuracy, completeness, readability, and adherence to templates and style guides, as defined by a client-driven scoring rubric
Continuous Improvement: Quality and delivery speed increased with each iteration through document roadmap refinement from POC to SaaS deployment
"We’ve seen clear improvements in both turnaround time and document quality. Each CSR gets faster, and the quality continues to improve. That compounding value is what gives us confidence to scale this approach.”
Lead Medical Writer, Top 20 Pharma